The results of this experimental study documents that fisetin and ALA, alone and in combination provides protective and analgesic effect against diabetes associated neuropathic pain. The effects of fisetin in this model has novel aspects as no chronic effect of this agent has been examined in the literature. ALA results are consistent with those previously reported
12.
In the present study we tested acute effects of fisetin and ALA, alone and in combination. For the chronic group, lower doses of the agents are used.
DNP is an important complication which has a high morbidity and mortality and deteriorates the quality of life of diabetic patients. Sensorial motor polyneuropathy is the most common type, also impairing quality of life especially due to diabetic foot ulcers. The use of rodents as a painful neuropathy model allows evaluation of both the electrical and neurochemical activities of the nervous system, as well as behavioral responses to sensory stimulants. The time the animal withdraws extremities upon exposing the tail or paw to heat is used as the method to determine hyperalgesia and hypoalgesia. Pain threshold responses contribute to obtaining indirect information on diabetic neuropathic pain. Hyperalgesia development in mice in which diabetes was induced with STZ starts on day 8 following STZ injection and continues for at least 4 weeks 14. STZ results in an increase in the action potential of C fibers in diabetic mice and induces impairment in pain threshold responses. There are several published studies measuring the changes in pain threshold in experimentally-induced animal models of diabetes. Thermal and mechanical hyperalgesia develop in experiments which induce short-term diabetes, whereas thermal and mechanical hypoalgesia are observed in those that induce long-term diabetes 15,16. Slowing down in nerve conduction in rats occur 6 to 9 weeks following diabetes induction 16,17. Time-dependent thermal pain threshold changes observed in experimental studies also reflect diabetic nephropathy symptoms observed in humans. Long-standing diabetes results in losses in peripheral nerves and an increase in nerve conduction rate 18. Plantar analgesiameter is a method to measure nerve conduction rate experimentally in animals. This is a thermal acute pain model to determine pain threshold by measuring the time of response the animal gives to heat stimuli 19. The duration of this study is 8 weeks, and statistically significant prolongation compared to pre-diabetes values, thus hypoalgesia, was seen with latencies in pain thresholds measured by plantar analgesiameter 8 weeks after diabetes induction.
Hyperglycemia is the main cause of physiological, neurochemical and behavioral changes in painful neuropathy of rodents. Glycemic control is the most effective method in the treatment of diabetic neuropathy. The Diabetes Control and Complications Trial (DCCT) indisputably demonstrated the importance of glycemic control in the development of neuropathy in type 1 diabetic individuals. With strict glycemic control, the risk of developing neuropathy is reduced by about 64% over 5-year follow-up 20. United Kingdom Prospective Diabetes Study (UKPDS), the largest and longest study on type 2 diabetes mellitus, demonstrated that blood glucose regulation corrected vibration perception 21. Glycemic control reduces the risk of neuropathy but it is not a definitive method of treatment. Therefore, many alternative medicinal products were introduced to market in attempt to find a solution to neuropathy. The pathogenesis of DNP has not been fully understood and there is still no effective, ideal treatment. Currently, principally symptomatic treatment is given for diabetic neuropathy. The drugs used for this purpose alleviates pain, whereas they have no activity on the disease course, which is the main treatment target.
Alpha lipoic acid is a potent radical scavenger and a part of the endogenous antioxidative protection system. Oxidative stress plays a central role in the pathogenesis of diabetic complications. Caused by free radicals in diabetic neuropathy, oxidative stress leads to endoneural hypoxia and nerve dysfunction. Hyperglycemia-induced oxidative stress induces programmed cell death of nerves. The role of oxidative stress in nerve damage has been studied in experimental diabetes and in diabetic subjects. An experimental DNP model induced by STZ demonstrated that ALA improved neural blood flow and improved decreased levels of GSH, one of the most important markers of oxidative stress 9. ALADIN-1, the first prospective, placebo controlled, randomized, double-blind study with alpha lipoic acid demonstrated that intravenously administered ALA decreased symptom scores and was well tolerated 22. Similarly, SYDNEY-1 study demonstrated that ALA infusion given 5 times a week for three weeks resulted in clinically significant improvement in scales that assess disorders and deficits associated with diabetic neuropathy, including nerve conduction rate and neuropathic disorder 23.
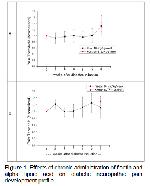
In our study, statistically significant latency was observed at all time points following administration of the drug compared to pre-treatment pain threshold values in both ALA groups, with the effect being more pronounced in the group treated with ALA 100 mg. Based on this result, it can be concluded that ALA corrected hypoalgesia in both groups with a more marked effect in the higher dose group. Hyperglycemia eventually leads to oxidative stress and reactive oxygen radicals (ROS) accumulation by interacting with a variety of pathways. The organism remains unaffected by free radicals as long as the oxidative balance is maintained. Studies showing the relationship between diabetes and its complications and reactive oxygen species emphasize that tissue damage increased free radical production and altered antioxidant defense system 24,25. Flavonoids were historically characterized on the basis of their antioxidant and free radical scavenging effects. Fisetin, a flavonoid derivative, protects nerve cell cultures from toxic stimuli such as ischemia and oxidative stress. Previous studies have shown that fisetin had several properties including anticancer, antiangiogenic, neuroprotective and antioxidation properties 26-28. In addition to its direct antioxidant capacity, fisetin also increases the levels of GSH, the major intracellular antioxidant. Glycation of macromolecules by reactive dicarbonyl and α-oxoaldehyde methylglyoxal (MG) is responsible for diabetic complications. Glutathione is a cofactor that is necessary for glyoxalase 1, the rate-limiting enzyme in eliminating MG. Fisetin also ensures maintenance of mitochondrial functions in the presence of oxidative stress. It further has anti-inflammatory activity against microglial cells and inhibits lipooxygenase. A study in diabetes-induced Akita mice demonstrated that orally administered fisetin reduces renal damage and protects the mice from anxiety-associated behaviors 29. The same study also showed that fisetin reduces the levels of oxidative stress markers thiobarbituric acid products (TBARS) and osteopontin. The effects of fisetin, with known oxidative effects, on pain threshold were examined in a DNP model because oxidative stress has an important role in the pathogenesis of diabetic neuropathy. Intraperitoneal fisetin at protocol-defined doses were administered to the mice with diabetic neuropathy, which was achieved through the study protocol. In the group treated with fisetin 3 mg, no significant changes were observed in pain threshold latencies measured 10, 30, 60 and 90 minutes after DNP was induced. In the group treated with fisetin 10 mg, on the other hand, shortened latencies, though not statistically significant compared to pre-treatment pain threshold latency values, were observed especially after 60 and 90 minutes. A study that induced experimental DNP investigated the effect of GSH in preventing and treating DNP. The study showed that the role of GSH in preventing DNP was only partial, and that it was ineffective in correcting sensory nerve conductions late after neuropathy has settled 30. In our study, late fisetin administration when hypoalgesia has developed and DNP has settled may explain the inefficacy on pain threshold. Oshawa et al. demonstrated that administration of acetyl-L-carnitine in diabetic neuropathic mice, in which hypoalgesia was induced, reduced pain thresholds and improved hypoalgesia to a statistically significant extent when it was given both with chronic administration before hypoalgesia development and at week 7, the time of onset of hypoalgesia 17. An other study showed that use of preventive low dose of fisetin for two weeks delayed the development of neuropathic hyperalgesia and allodynia in diabetic mice 11. Similar to this study, the observed effectiveness of ALA on hypoalgesia that define the late period of DNP may be due to the involvement of several different mechanisms in the pathogenesis, including those which have been described and those that await elucidation. In a mouse model of neuropathic pain fisetin can correct thermal hyperalgesia when administred orally and chronically. This action is time dependent since it is sensitive to thermal stimuli but not mechanical stimuli and is present following chronic rather than acute fisetin treatment 12. In our study fisetin was administred after hypoalgesia occurence and administration period was very short. The acute administration of fisetin may explain its ineffectiveness.
Although we did not measure mortality, some animals were lost due to very high blood glucose levels and no standart therapy (no insulin being given). But, the level of mortality was lower in ALA received group, which indicates an advantage of this agent which is already in clinical use as adjuvant.
In conclusion, neuropathic pain is a heterogeneous condition with different causes and mechanisms. Various treatments have been used in DNP but a prototype drug or a method has not yet been found. New treatment protocols will be discovered once the pathogenetic mechanisms has been elucidated. It should be borne in mind that treatment efficacy observed in experimental diabetes may be different from that seen in human, and that the effect of diabetes on nervous system in humans is more extensive, chronic and serious. Therefore, novel pharmacological agents and new treatment methods that target the pathogenesis of DNP are needed.