It is known that activated inflammatory cells lead to ROS production in BD an autoimmune disease
3-7. Increased ROS production, in turn, enhances LPO products and causes tissue injury
3-7. It has been reported that serum levels of malondialdehyde (MDA)
5,10,36, and LOOHs
6, which are LPO products, increase in BD, and high serum MDA levels negatively correlated with antioxidants
10 and treatment with vitamin E, which is an antioxidant, significantly reduces MDA level
5. Similarly, it has been established that plasma and erythrocyte levels of thiobarbituric acid reactive substance (TBARS), an LPO product, are high in BD and there has been no significant difference between active and inactive BD groups in terms of TBARS levels
37. However, Orem et al.
3 have reported that LPO products in the active BD group are higher than those in the inactive BD group. In the present study, the level of LOOHs which are byproducts of LPO have been found higher in the BD group, too. Although when compared with inactive BD group, the level of LOOHs has been found slightly higher in the active BD group, this difference has been not statistically significant. This result we obtained is consistent with the study of Akar et al.
37. The inconsistency of the results about LPO products in active and inactive BD groups might be related with the differences in the methods of measurement used and/or in the methods used to determine the disease activity. It is known that currently there is no agreed activity criterias for BD.
CP, which is synthesized by hepatocytes and which carries 90-95% of copper, is a protein with antioxidant characteristics 24. Oxidation of increased amounts of plasma homocysteine restores CP's redox state, leading to decreased copper transport into the cells 25. Previous studies have shown that CP is a powerful plasma antioxidant, when iron-stimulated reactions are involved and this has mainly been ascribed to its ferroxidase activity. The conversion of Fe2+ into Fe3+ can decrease oxidation by blocking the Fenton reaction through a decrease in the quantity of oxidant Fe2+ or sequestration of iron from apotransferrin 26. Human CP has recently been ascribed a thiol-linked peroxidase activity which can remove H2O2 and LOOHs 24. On the other hand, it has also been reported that ROS inhibit CP activity 28. It is noted that CP activity increases in BD and this is related with the acute phase response of CP 7,22,23. In the present study, however, it has been found that CP activity is reduced in BD and that CP negatively correlates with CRP, a marker of acute phase response, and positively correlates with SH which is known as antioxidant. CP has also antioxidant properties 24-26. The decrease in CP activity along with other antioxidants in BD supports this claim and it seems to be an expected result. However, it is also possible that increased oxidants may be responsible for the decrease in CP activity, as stated by Gutteridge et al. 28.
Serum SH groups act as important cellular scavengers of peroxides and so help to protect cells from damage by these molecules. Decrease in SH level not only impairs cells' response to oxidants, but also changes the functions of inflammatory cells 38. It has been reported that in BD there is a decrease in SH level 7,22,23, which negatively correlates with MDA and CRP levels 22, and which is more marked in active BD, than in inactive BD 7. In our study, SH level has been found to be lower in the BD group, in comparison to the healthy controls, but there was no statistical difference between the SH levels of active and inactive BD groups. The positive correlation between the SH level and the activity of CP, another antioxidant, seems to support the claim that LPO products, together with other antioxidants, reduce SH levels. Lack of a significant difference between active and inactive BD groups with regard to SH levels may have resulted from the inadequacy of criteria used to determine the disease activity.
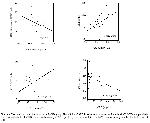
It has been reported that PON1 activity decrease in BD 36 and RA 39,40 cases, when compared with the healthy individuals, and activity of ARE also decreased along with PON1 in RA cases with amyloidosis complication 41. Activities of PON1 and ARE enzymes have been found to be lower in the BD in the present study, too. It has been shown that PON1 hydrolyzes LPO products and H2O2 42. LPO products are not only formed via lipoprotein oxidation, but also lipids in the cell structure which undergo peroxidation in oxidative stress 18. It is known that LPO products increase in BD 3,5,6,10,36,37. In our study, a negative correlation has been found between ARE activity and LOOHs levels. We think that the decrease in the activities of PON1 and ARE enzymes may be important in terms of the progression of BD.
PON1, which is a part of HDL cholesterol, is a strong antioxidant enzyme that is believed to have a protective role in the atherosclerotic process, by both contributing to HDL's protective effect against atherosclerosis and preventing lipoprotein peroxidation and oxidation of LDL cholesterol 18-20. Shih et al. 21 have demonstrated that rats with genetically PON1 deficiency are prone to atherosclerosis. It has been established that the activities of PON1 and ARE decline in atherosclerosis 18-20. Endothelial dysfunction and accelerated atherosclerosis have been emphasized in BD 4. In the light of this data, besides its contribution to disease progression, the decrease in PON1 and ARE enzyme activities suggest a possible increase in atherosclerosis incidence, in BD.
It is known that PON1 structure includes three cysteine residues carrying sulphydryl groups. Of these, cysteinyl residues on the position 41st and 352nd are involved in the formation of intramolecular disulphide bonds, while cysteinyl residue on the position 283rd is free and responsible for activity 18-20,43. Aviram et al. 43 have demonstrated that LPO products are bound to cysteine residues found on the 283rd position of PON1, thereby inactivating PON1 and ARE activities. The negative correlation found between LOOHs level and ARE activity in our study supports the idea that LPO products are responsible for the decrease in the activities of PON and ARE in BD.
Experimental studies have shown that cytokines like IL-1 and TNF-α reduce the production and the activity of PON1 44,45. It is known that the serum levels of many cytokines like IL-1, IL-6, IL-8 and TNF-α increase in BD 46-48. One probable reason of the decrease in activities of PON1 and ARE in BD might be the increase in these cytokines as pointed out by Feingold et al. 44 and Kumon et al. 45. Not finding significant correlation in PON1 and ARE activities with ESR, CRP and WBC values in our study seems to decrease the above probability. Activities of PON1 and ARE are known to be affected from HDL level 18-20. Although we have observed a positive correlation between HDL level and activity of ARE, not finding significant difference in HDL level between BD and healthy control reduces the probability of only HDL to be responsible from the decrease in activities of PON1 and ARE in BD.
In conclusion, it has been established in the present study that the level of LOOHs, LPO product, have increased, and various antioxidants have decreased in the BD group, relative to the healthy control group. PON1 and ARE, which have antioxidant characteristics, are also known to possess protective effects against atherosclerosis. The decrease in the activities of these enzymes may have part in the development of atherosclerosis, as well as, tissue injury in BD. It is necessary to evaluate atherosclerosis in BD and to determine the role of decrease in antioxidants in atherosclerosis by controlled, prospective and multi-centered studies in large series.