Free radicals play a role in a variety of diseases and it has become apparent that pathogenesis of many diseases can be lightened by means of understanding cell sources of free radicals and defence mechanism against free radicals. Free radicals are originated from activated phagocytes, antineoplastic agents, irradiation, addictive drugs, stress, otooxidation of small molecules, enzymes, proteins, electron transport systems of mitocondrium, membran of plasma and conditions of oxidative stress
1,2,4,7. Free radicals effect metabolism of cells through the damaging effects on the metabolism of protein, DNA, carbonhydrate, lipids, enzymes and other molecule groups
5,10,11,21,22.
The protective effect of Vitamin E (alpha tocopherol) might be attributed to a structural effect, however, and there is an evidence from experiments upon cell cultures that the presence of vitamin E can affect the types of fatty acids that become incorporated into membrane lipids22. Keskin et al.20 and Göktürk23 investigated the effect of alpha tocopherol on the healing of bone in rabbits and rats respectively. In this study, it was to investigate the effect of alpha tocopherol on the healing of bone fracture in dogs.
Vasoconstruction and temporary ischemic period are developed in fracture site when a bone fractured, an arterial vasodilatation and reperfusion in fracture sites are then observed. Polymorphonuclear leucocytes, macrophages and mast cells are migrated to fracture sites in the first 5 days of fracture. This phase is important for fracture healing. It is believed that free oxygen radicals produced through the activation of polymorphonuclear leucocytes damage granulation tissue and retard wound healing6,20,23-25. Negative effects of free oxygen radicals on the fracture healing were reported8,15,17,26. Norazlina et al.9, reported that Vitamin E deficiency may cause loss of bone calcium in growing female rats. Hodis27 has mentioned that for normal antioxidant effect 400 IU vitamin E per day and for maximum antioxidant effect 800 IU per day should be administered 1000 IU per day is consider as megadose27. Some other authors28,29 have suggested a prophylactic dose of 1000 IU per day for 3-4 months to decrease coronary health problems. Keskin et al.20 and Durak et al.24 have administered 20 mg/kg/day alpha-tocopherol in rabbits. For that reason, in this study 20 mg/kg/day dl-alpha-tocopherol acetate was injected intramuscularly to treatment group for one week as the first 5 days of fracture is important for fracture healing2,20,23,24.
A role of free radicals has been proposed in the toxicity of numerous chemicals and in the pathogenesis of many diseases30. An extensive list of disorders in which free radicals are implicated is still growing, at least in part because these reactive molecules can produce most of the tissue changes that have been identified during a variety of injurious processes3. Some substances defined as antioxidants are used to either prevent formation of or to scavenge free oxygen radicals and their damages. Dl-alpha tocopherol is most active antioxidant among the tocopherols. Vitamin E prevents oxidation of other molecules by means of easily being oxidated31-33. Free radicals levels were not analized in this study because the aim of this study was to investigate the effect of vitamin E administration on the healing of fracture clinically, radiologically and histopathologically.
Vitamin E deficiency would increase lipid peroxidation. It has been shown that lipid peroxidation enhance bone resorption by directly activating osteoclasts8,9,34,35. Avitabile et al.36 reported that an association between low activity of antioxidant systems and demineralization of bone, consequent upon enhanced free radical levels. Yee and Ima-Nirwana37 reported that exposure to an oxidizing agent, ferric nitrilotriacetate, reduced bone calcium content, and that this was prevented by vitamin E supplementation. Therefore, it is suggested that the vitamin E deficiency increased free radical activity, thus enhancing bone resorption and demineralization, which was seen as significantly low bone calcium content. Cohen and Meyer38 found that vitamin E and selenium deficiency predispose rabbit bones to osteomalacia and decreased the biomechanical strength of the bones. However, vitamin E supplementation was protective against bone loss due to rotational stress in rats38. Sergeev et al.39,40, found that rats with a vitamin E deficiency had decreased absorption of calcium through the intestines and kidneys, as well as decreased deposition of calcium in bones. Similar to these results, results of current study showed that it is clear that vitamin E plays a role in normal bone mineralization, either by its antioxidant effects or by increasing calcium availability for bone deposition.
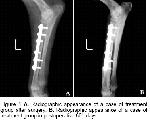
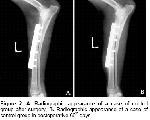
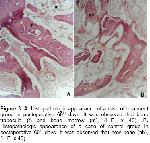
The clinic, radiographic and histopathologic findings of the present study shows that in order to prevent negative effects free oxygen radicals on osteogenesis the administration of 20 mg/kg/day dl-alpha-tocopherol, a high antioxidant feature appeared to have a usefull effect on early healing processes (first fifteen days) of osteogenesis in experimentally induced fracture dog model.