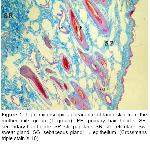
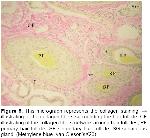
General structure of skin is affected by factors such as climate, caring-feeding conditions, age and gender. Especially the race of the animal affects skin structure to a significant extent
12. In all sections examined, it was determined that skin is generated in epidermis and dermis layers which are coherent with the skin structure and features of domestic mammals
2,5,13,14. Kurtdede et al.
6,15 observed that epidermis of Awassi sheep was thicker in summer compared to other seasons. The average epidermis thickness was determined as 42 µm in Merino rams
13, 21.2 µm Akkaraman sheep
7,16, 12.7 µm in Dağlıç sheep, and 21.32 µm by in Karacabey Merino sheep
14. In the present study, the thickest epidermis was found 40 µm in the 2
nd group. In addition, str. corneum was found thicker and, mitotic figure in str. basale was observed in less density compared to other two groups. This situation could be depend to inadequate feeding of lambs.
Dermis, the essential layer of the leather used in industry, consists of two sub layers. The average thickness of papillary layer was reported as 1755 µm in Akkaraman sheep, 1149 µm in Dağlıç sheep7,16,17, 1614.58 µm in Kıvırcık sheep, 1490.97 µm in Karacabey Merino sheep14, 1790 µm in Awassi sheep, 2150 µm in Sakız sheep4. In this study, the papillary layer was determined as the thickest in the 1st group with 2151.25 µm and thinnest in 2nd group with 1906.25 µm. This condition could be depend to the thickness of papillary layer in the 1st group to loose structure in connective tissue and its thinner in the 2nd group to inadequate feeding.
The thickness of reticular layer was determined as 968 µm in Akkaraman sheep, 808 µm in Dağlıç sheep7,16,17, 1247.92 µm in Kıvırcık sheep, 1415.97 µm in Karacabey Merino sheep14, 880 µm in Awassi sheep, 944 µm in Sakız sheep4. In their study of the dermis of skin samples, the reticular layer was determined as the thickest in the 1st group lambs with 1033.75 µm and thinnest in 3rd group lambs with 863.13 µm. This situation could be depend to inadequate feeding of lambs.
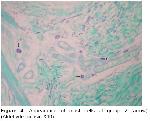
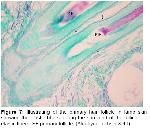
It is important to obtain information about the collagen fiber orientation in biological fibrous tissues such as human and animal skins because the collagen fibers orientation in the skins may be closely related to the motional functions of the body. However, no reports are yet available on the distribution of collagen fiber orientation in a whole skin18. Özfiliz et al.2,14 observed papillary layer where thin collagen fibers and epidermal formations are present, whereas reticular layer where thicker collagen fibers form clusters and no epidermal formations exist. Dağlıoğlu and Bayramlar4 reported that the collagen fiber clusters were observed to have the thickest in surface portions of reticular layer in dermis. We can also say that, in our study, collagen fiber get thicker from papillary layer towards reticular layer and form larger clusters in all groups, and that this is consistent with the results reported by Özfiliz et al.2,14.
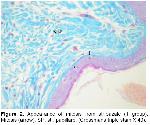
Humans, mice, deer, cows, horses, pigs, and some kinds of dogs typically have this type of hair arrangement, with single, uniform hair follicles, each in communication with other hair follicles through long elastic fibers that extend out through the matrix19. Starcher et al.19 have earlier observed that elastic fibrils in mammal skins extend just under the epithelium parallel to each other and from one hair follicle to another; elastic fibers are attached from one follicle to another especially in deer skin and the elastic fiber array surrounding the hair follicles in sheep skin is very similar to that of the rabbit. Kurtdede et al. stated that in Lincoln, Akkaraman and Awassi sheeps, elastic fibers surround follicle weakly at the line of sebaceous glands, whereas elastic fibers surround primary and secondary follicles strongly at the bottom. Konya Merino, they surround follicles strongly at sebaceous glands level and at the bottom6,15. Özfiliz et al.2,14 observed that elastic fibers concentrate around sebaceous glands in Merino sheep. Dağlıoğlu and Bayramlar4 reported that elastic fibers that extend parallel to surface and each other are present in papillary layer of Awassi sheep. In our study, papillary layer elastic fibers are consistent with the results of Starcher et al.19, Dağlıoğlu and Bayramlar4.
Özfiliz et al.2,14 reported that reticulum fibers of Awassi sheep are located around sweat glands and hair follicles, which is consistent with our results.
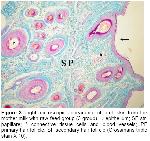
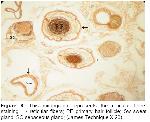
Wool-bearing animals and animals with fine fur have two or more dissimilar types of hair follicles. Included in this group are the rabbit, sheep, fox, squirrel, raccoon, some kinds of dogs, and many other mammals19. The type and arrangement of follicles in the skin of all sheep regardless of breed are similar. The follicle population typically consists of a basic group of three primary follicles arranged in rows and a variable number of secondary follicles lying on one side of the trio of primaries. All primary follicles are associated with a sweat (sudoriferous) gland, an arrector pili muscle and a sebaceous gland, while secondary follicles possess a sebaceous gland only9. Corbett20 concluded that it was unlikely that maturation of primary follicles could be affected by malnutrition without also causing the death of the fetus and the dam. In examined sections, primary follicles in groups 2 and 3, secondary follicles in groups 6 and 8 and a sweat and a pair of sebaceous glands located just on the side were seen in str. papillare.
The ratio of the total number of secondary follicles (S) to primary follicles (P), or S/P ratio is a measure of the potential number of wool fibers that can appear under favorable conditions9. Regardless of the experimental method used, these studies found that prenatal nutritional stress influences only the secondary follicle population. This is in agreement with Corbett20 who concluded that the primary follicle population is unaffected. There is however disagreement among the published authors as to whether the prenatal effects on the secondary follicle population are permanent9,14 or transitory9,21. This disagreement can be the result of three possible causes; namely breed differences, the severity of the nutritional stress and the timing of the nutritional stress. In our study, the number of primary and secondary follicles per square millimeter in the 2nd group was more than other groups.
In conclusion, there was no difference in terms of the distribution of the connective tissue fibers and the density between the groups, for this reason, nutrition is not effective in this aspect. The groups to which were given mother milk had the thickest dermis layer and lesser hair follicle; this may be said to be important in leather industry.