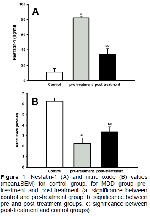
We examined the effects of three months venlafaxine
treatment on nesfatin-1 level in MDD patients for the first
time. Venlafaxine is more effective antidepressive drug
especially in resistive depressive patient's treatment
14.
As shown in a previous study, nesfatin-1 level was found
to be markedly high in MDD patients
8. In this study, we
clearly showed that nesfatin-1 decreased significantly
after treatment. However, it should be strictly emphases
that, this post-treatment nesfatin-1 value is still higher
than the normal subjects' levels (
Figüre). This may be
related with the duration of the MDD treatment. To clarify
this topic, further studies, concerning the effects of long
treatment period on nesfatin-1 level in MDD patients, are
necessary. It is also important to emphasize that after
three months treatment the patients depression status as
classified by HAM-D scale improved but not to normal
levels
8. Because, one may think that the prognosis of
the patients could be related the level of nesfatin-1
4.
In addition, high nesfatin-1 level has been reported in
generalized epilepsy
7 and in panic disorders
compared to healthy subject
15.
The close relationship between nesfatin-1 and central
nervous system hormones has been reported16.
Importantly, the interaction of nesfatin-1 and central
nervous system hormones has an important role in
etiology of depression8. A markedly high nesfatin-1
level found in raphe nucleus neurons and locus
coeruleus neurons of stressed rat compared to nonstressed
rat17. This is important that locus coeruleus
neurons have a powerful regulatory role on many mental
functions known to be dysregulated in MDD18. In
addition, raphe nucleus, which is the main source of
serotonergic innervations in the brain, is the primary
neurotransmitter accused for some metabolic and
psychiatric changes including, appetite, energy, sleep,
mood, libido and cognitive function changes in MDD19.
It is suggested that an increase in nesfatin-1 level
may result an increase in anxiety via affecting
melanocortin system20. In clinical medicine, nesfatin-1
level could be important criteria to describe the patient
prognoses and effectiveness of treatments. In previous
study performed in epileptic patients, significant
decreases in nesfatin-1 level reported following anti
antiepileptic treatment7.
It has been suggested that ghrelin and nesfatin-1
could be closely related in ethiopathologies of psychiatric
diseases in addition to their effects on appetite and food
intake regulation7,10. It is well known that ghrelin and
nesfatin-1 is adversely affecting hormones21. The
anorectic effect of nesfatin-1 has been shown after
directly administering it into the brain of rodents1. In
literature, it is well known that ghrelin increases food
intake in normal condition3,10.
The results of a study performed in rodent suggested
that ghrelin has antidepressive and depressiongenic
effects22. In this study, basal ghrelin level of MDD
patients was markedly lower than the control group,
which could be the results of the depression7,10. The
lower ghrelin level before and after treatment could be
related with the low antioxidant capacity, which is know
to be reduced in depressive patients. Importantly, it has
been shown that ghrelin has important effects on
neuroprotection23.
Despite the decrease in ghrelin level after treatment,
we have observed increase in body weight (Table 1). It
might be expected decrease ghrelin level results
decrease in body weight rather than increases. The
increased body weight during treatment period may be
related with the reduced nesfatin-1 level or direct effects
of drug affecting metabolic rate24. In addition, it is also
be emphasize that NO is an important regulatory factor in
food intake25. It is known that basal metabolic rate is
one of the important factors in body weight regulation.
The main reasons for weight gain in MDD patients after
treatment are increased caloric intake, reduced energy
expenditure or a combination of both. Venlafaxine may have important effects on basal metabolic rate and
physical activity26.
In this study, serum NO levels, before and after
treatment, were measured and it was found to be
markedly low in depressive patients. Importantly, NO
level increased significantly after treatment, but it was
still lower than the control levels. In depressive patients,
end-production of NO metabolism has been shown to be
high in some studies27. In contrast, low NO levels in
depressive patients compared to normal subjects has
been reported28. Interestingly, increased NO levels
results a suicide attempt especially in mood disorders
and schizophrenia patients29. Thus, evaluation of NO
level could be important criteria in depressive patients
prognosis27. However, the clear and satisfactory
explanation in NO level and depressive patients has not
been documented yet.
In this study, three months of venlafaxine treatment
associated with significant improvements in nesfatin-1
and NO levels compared to their basal values over a
three months period. In this regard, nesfatin-1 and NO
could be useful markers to evaluate the effectiveness of
treatment and prognosis of MDD in addition to the other
parameters. However, further studies with a large
number of sample size and with a longer treatment
duration are required to understand the factors affecting
nesfatin-1, ghrelin and NO metabolism and their roles in
the etiology and also in prognosis of MDD.
Acknowledgement
This study financially supported by FUBAP (Firat
University Scientific Research Support Unit, Project No:
1878) for master project.