RIPK4 is a critical regulator of keratinocyte differentiation whose deficiency in mice and human shows similar congenital abnormalities associated with abnormal epidermal development
5-7. So far, RIPK4s role in epidermal differentiation was studied either in keratinocytes obtained from RIPK4 (-/-) knock-out mice or in immortalized keratinocyte cell lines
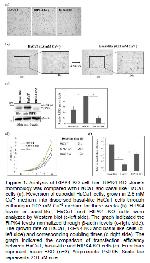
8,9. In keratinocyte cell lines based studies, RIPK4 was transiently depleted by transfecting cells with RIPK4 targeting si-RNAs which restricts the long term analyses of RIPK4 mediated events including keratinocyte differentiation. Therefore, in this study, RIPK4 KO clone in which RIPK4 expression was stably depleted using CRISPR/Cas9 method was analyzed. Interestingly, following the stable depletion of RIPK4, RIPK4 KO cells displayed scattered and rounded morphology, which was quite different from cuboidal HaCaT cells that show a tendency to grow in clusters (Figure 1a). Calcium is critical for the differentiation of basal layer localized keratinocytes
15. It is possible to revert the differentiated HaCaT cells back to the undifferentiated basal layer localized progenitor cells, which are referred to as basal-like state cells, by growing them in low calcium condition continuously. These progenitor cells can re-differentiate simply by switching on high calcium medium
11,12. Interestingly stable depletion of RIPK4 expression in RIP4 KO cells, which was fivefold compared to parental cell line, shifted these cells into a basal-like state, even though they were grown in regular calcium-rich medium (Figures 1 and 2). As previously mentioned in Sumer et al., 2019
13, RIPK4 KO carries compound heterozygote RIPK4 mutations. One of the mutations is one nucleotide insertion that resulted in a premature stop codon and the other one is 12 nucleotides deletion. Even though 12 nucleotide deletions still lead to the expression of protein, the 5 fold suppression of RIPK4 expression compared to parental cell line indicated that deletions induced destabilization of the protein (Figure 1c).
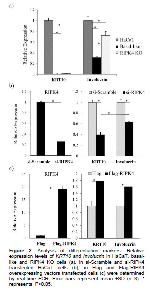
Considering the morphological resemblance to basal-like state cells, we compared RIPK4 KO with basal-like state cells along with HaCaT cells in terms of distinctive features associated with basal-like state cells such as enhanced growth rate, increased transfection efficiency and reduced level of keratinocyte differentiation markers 11,12. Similar to basal-like state cells, RIPK4 KO cells showed enhanced growth rate (Figure 1d) and transfection efficiency (Figure 1e) compared to HaCaT cells. Previously, it has been demonstrated that protein levels of KRT10 and involucrin decreased in basal-like state HaCaT cells compared to HaCaT cells and the fold reduction was more prominent in KRT10 than involucrin 11. In accordance with this report, both KRT10 and involucrin expression were reduced in RIPK4 KO cells and it was more apparent for KRT10 (Figure 2a). In parallel with the morphological resemblance, the depletion of RIPK4 shifted HaCaT cells more toward basal-like state cells in every aspect. However, in RIPK4 KO cells the extent of differences compared to parental cell lines was not as high as basal-like state cells (Figures 1 and 2). Indeed continuous depletion of RIPK4 in HaCaT cells turned these cells into an intermediate state between undifferentiated basal-like and differentiated HaCaT cells.
In the epidermis of RIPK4-/- mice, due to the differentiation-induced growth arrest delay at suprabasal localized cells, stratified layers organization and associated layer-specific keratinocytes markers expression are disrupted 5,16. In accordance with, the primary keratinocytes obtained from RIPK4-/- mices skin is unable to differentiate upon stimulation with epidermal differentiation factors such as calcium and vitamin D 16. Similar to keratinocytes obtained from RIPK4-/- mice, the depletion of RIPK4 expression strongly inhibited KRT10 and involucrin expression levels in RIPK4 KO and si-RIPK4 transfected cells even though they were grown in high Ca+2 medium (Figures 2a and b). Moreover, basal-like state cells showed reduced RIPK4 expression compared to HaCaT cell as well (Figure 1c). These results indicated that RIPK4 is one of the important determinants for de-differentiation process which possibly requires additional Ca+2-mediated factors for the full transition.
When RIPK4 was transiently depleted by RIPK4 targeting si-RNAs in HaCaT cells there was no obvious difference in terms of morphology (data not shown) indicating the long term depletion is required for the appearance of this phenotype. This situation can also be explained with the level of KRT10 suppression, which is quite high in basal-like state and RIPK4 KO cells (90 fold and 55 fold respectively) compared to si-RIPK4 transfected HaCaT cells (2.5 fold). KRT10 is an early keratinocyte differentiation marker and a critical determinant of basal to suprabasal layer transition 17. Interestingly, RIPK4 depletion shows its effect profoundly on the KRT10 level, which emphasizes RIPK4s role in the early differentiation process. In accordance with, in our previous study, it was shown that RIPK4 interacts with basal layer-specific keratin14 (KRT14) which localizes just below KRT10 expressing spinous layer 13. In addition to the levels of differentiation markers, RIPK4 KO exhibited this intermediate phenotype between basal-like state cells and HaCaT cells in terms of transfection efficiency and growth rate as well (Figures 1d and e).
As a conclusion, within this study, a RIPK4 knocked-out clone, which exhibit an intermediate differentiation state between basal-like state and HaCaT cells, was characterized. Indeed, this particular characteristic makes this clone an appealing in vitro model to understand the molecular basis of basal to suprabasal layer differentiation. Besides, with this study the previous in vivo mice based studies that show the correlation between RIPK4 depletion and suppression of epidermal differentiation 16,18 were confirmed in HaCaT cells. Moreover, in accordance with hypothesis, continuous depletion of RIPK4 was shown to be necessary to observe the distinct effect of RIPK4 on keratinocyte differentiation.
Acknowlegements
I thank Ceren SÜMER and Asiye Büşra BOZ ER for their technical support in western blotting and real-time PCR experiments.