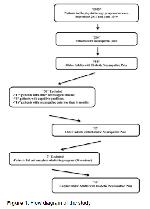
This study was conducted at the Hacettepe University. The University Ethics Committee approved the study (GO 17/622).
All older adults with diabetic neuropathic pain patients who were eligible and recruited to the physiotherapy program between September 2017 and June 2019 were considered for inclusion in this retrospective analysis. Identified data for the initial assessments and the final assessments (referred to as discharge) were extracted from medical records by a staff physical therapist. Because of the retrospective nature of the study, discharge data were only available for older adults with diabetic Neuropathic pain patients who completed the entire 30 exercise-sessions (Figure 1).
Patients entering the physiotherapy program had to be diagnosed as having Diabetic Neuropathic pain to join the program by a neurologist. Once the diagnosis was obtained, patients were scheduled for a physical therapy evaluation. Afterward, patients joined the program on a rolling basis and typically attended 5 sessions per week until they reached a total of 30 sessions. The minimum amount of time to complete the program was 6 weeks; however, if a patient missed a session because of illness, hospitalization, appointment, or other personal conflicts, the patient continued in the program until all 30 sessions were completed.
The information of the patients who met the inclusion and exclusion criteria were recorded from the patient files. Inclusion criteria were; having type 1 or 2 diabetes, having symptoms of neuropathic pain for at least 6 months, over 65 years of age, having pain that was resistant to medical treatments. Exclusion criteria were; having any other neurological disease (e.g. cerebrovascular ischemia), having cardiac arrhythmias or cardiac pacemakers, myocardial infarction within the past 6 months, untreated hypertension, infection or gangrene, history of vascular insufficiency in the legs, drug or alcohol abuse, psychiatric disease, major organ disease, Patients taking medication that may influence neuropathic symptoms (such as α-lipoic acid, tricyclic antidepressants or anticonvulsants), and patients with ulcers, amputations caused by ischemia, or cancer diseases were excluded.
Extracted data comprised of the following data routinely collected in medical or PT records: The demographic characteristics, diagnoses, the date on which the pain started, and the accompanying complaints. Also, about Neuropathic pain, the neuropathic component and density of the pain were evaluated with the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain questionnaire, and the patients whose pain levels were 12 and over were included in the study, which is in accordance with the literature (14). The words that the patients used to describe the pain quality were recorded (burning, paresthesia, tingling, etc.). The other assessments were listed below;
Characteristics of Pain (LANSS Pain Questionnaire): This is a multi-dimensional scale used in discriminating the neuropathic and nociceptive pain from each other and is based on the analysis of the data from the questionnaire that can be applied while the patient is in a bed. The LANSS consists of 7 items, 5 of them questions pain symptoms. The other two are directed to sensory examination that includes allodynia and pin-prick test. Answers to these questions are as Yes/No. The scale was scored between 0-24, and the points above 12 made the physician consider the Neuropathic pain 14. The validity and reliability procedures of LANSS in Turkey were performed by Yücel in 2004 15.
Pain Localization (Body Diagram): The patients were asked to mark the painful area on a body diagram. If there were more than one painful areas, these areas were marked with a different colored pen; however, the most painful area was taken into consideration in the treatment 16.
Pain Severity (Visual Analog Scale): The pain levels of the patients were evaluated using the Visual Analog Scale (VAS). The patients were asked to consider their pain levels for the past 2 weeks and mark the average, the most severe, the least severe, and current pain levels on a horizontal line 17.
Health-Related Quality of Life (Nottingham Health Profile): The Nottingham Health Profile (NHP) was used in evaluating the quality of life of the patients. This scale is a general quality of life scale that aims to measure the self-perceived health status in terms of physical, emotional, and social aspects 18. The NHP is also a general health questionnaire that has 6 sub-parameters under the headings, the Energy Level; pain; emotional reactions; social isolation; sleep; and physical activity; and which was answered as Yes/No. Each sub-parameter was between 0 and 100 points; the total points varied between 0-600 points 18. Its Turkish validity and reliability process was performed in 2000 by Ayşe Küçükdeveci and has used in patients with neuropathic pain 19.
Intervention: The patients who were included in the study received TENS application for 6 weeks, 5 days a week, 20 minutes a day in 30 sessions total. The application was performed with the Cefar Active XT TENS device which produces 2-channel, 4-sockets, asymmetric, biphasic square wave. In the application, the frequency of the square wave was set to 80 Hz beat width for each second; the beat width to 350μs, and the current amplitude was set to 0-100 mA. The electrodes of the device (dura-stick plus 5*5 cm. cable, smooth needle pin sticky electrode) were set to face the upper part of the painful area. The current amplitude was kept at a level that would not cause/increase pain in the patient by considering the sensory threshold of the patient.
Strengthening (trunk flexion-extension) and stretching exercises (stretching and elongation), mat activities (bridging), functional activities (weight transfer from anterior to posterior and left to right), and range of motion exercises (trunk flexion, extension, left-right rotation, lateral flexion) were performed by the patients. The exercises were applied carefully not to cause pain and tiredness in patients.
Average pain severity was used for calculating sample size. The effect size was described with post hoc analysis as 0.87 by using means, standard deviations. The power of the study was calculated from the Gpower 3.0.10 analysis program and was found 88% for 16 individuals.
Statistical Analysis: The statistical analyses of the study were made by using the SPSS 15.00 Statistical Package Program. The variables which were defined by measurements were expressed as median (25-75% interquartile range (IQR)), and percentage (%) was used for the variables that were defined by counting. The statistics of the changes between the pre-treatment and post-treatment values within and outside the groups and the areas were evaluated by using the Wilcoxon test.