The present study revealed that RES suppresses cranial RT-induced microglia-mediated neuroinflammation by promoting autophagy in rats.
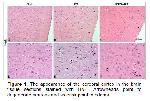
Neuroinflammation within the central nervous system is the inflammatory reaction of microglia, and to a lesser extent of other glial cells, and neurons against infectious agents, trauma, ischemia, stroke, cranial radiation, etc. 26-28. In the present study, it was determined that RT administration significantly increased CD68-positive cell density. Consistent with this result of the present study, Ruddy et al. also reported that cranial irradiation promoted an increase in microglia proliferating 29. CD68, a microglial marker, is a membrane protein expressed by monocytes and macrophages that remarks phagocytic activity 30. It has been reported that cranial RT potently induces neuroinflammation, with markedly increased activated microglia 31.
Microglia, the resident immune cells of the central nervous system, play a crucial role in immune responses and the maintenance of neuronal tissue homeostasis. It also responds to changes in the microenvironment by upregulating various cell surface receptors and producing a wide variety of secreted factors. There is a balance between pro- and anti-inflammatory factors in the neuronal tissue under normal physiological situations. Whereas, a shift toward the pro-inflammatory state occurs by disturbing the balance after exposure to foreign conditions and substances such as radiation, infectious agents, trauma, and ischemia. Although microglial activation is necessary for protection under abnormal conditions, such activation may cause neuronal injury and death beyond a certain threshold 32,33. Microglia abnormally activated by radiation continuously produce neurotoxic cytokines such as TNF-α, IL-6, and IL-β1 3. In the current study, the effects of radiation on TNF-α expression were investigated and it was observed that radiation application significantly increased TNF-α expression.
TNF-α is able to be produced by other glial cells and neurons, but it is accepted that microglia are the main source of this cytokine during neuroinflammation. TNF-α, a proinflammatory cytokine, has both homeostatic and pathophysiological roles in the neuronal tissues. In pathological states, a large amount of TNF-α is expressed by microglia. This de novo expression of TNF-α is a significant indicator of the neuroinflammatory responses 28. TNF-α and other cytokines released from macrophages affect blood-brain barrier permeability, leukocyte adhesion, and microvessel diameter and cause predominantly acute RT-induced neurodegeneration including gliosis, edema, and neuronal cell death 34. Consistent with the literature, in the present study, it was also determined that the number of degenerate neurons was significantly higher in the RT group. Consistent with current results, it has been reported in many studies that radiation exposure causes neuronal damage and cell death 3,35.
Increasing evidence has shown that RES exhibits multiple bioactivities, including anti-inflammatory, anti-oxidant, anti-apoptotic, and anti-aging effects 10,36,37. The current study demonstrated that RES has neuroprotection against RT-induced neurotoxicity. These neuroprotective effects of RES might be depended on the suppression of microglial activation and the subsequent decrease in the release of various neurotoxic cytokines such as TNF-α, IL-6, IL-1β, and NO. RES is able to decrease the release of pro-inflammatory factors through the inhibition of the NF-κB signaling pathway 10. Consistent with these results, the present study demonstrated that RES administration significantly decreased the intensity of CD68 and TNF-α positive cells. In addition, in the current study, it was determined that RES significantly reduced the increased number of degenerate neurons caused by RT. It has been reported that microglial activation may cause neuronal death by releasing neurotoxic pro-inflammatory molecules 14. Therefore, data of the current study indicate that the administration of RES exerted a significant anti-inflammatory effect by inhibiting microglia activation and subsequently reducing TNF-α expression.
It has been also reported that the anti-inflammatory effects of RES may be related to its stimulation of autophagy 16. Extensive research has documented that autophagy has substantial neuroprotective roles in many neurodegenerative disorders. The studies have shown that deletion/mutation of the autophagy-related genes disrupts cellular homeostasis, resulting in some neurodegenerative diseases 38-40. Autophagy, a crucial homeostatic process for cell survival, is the main regulatory catabolic mechanism by which cells digest and recycle cytosolic material, including impaired macromolecules or damaged organelles. In this process, the degraded cytosolic material is surrounded by a double-membrane vesicle called an autophagosome and which then fuse with lysosomes. After fusion with lysosomes, the autophagosomes' content is cleared up and then the remaining products may recycle for the bioenergetics metabolites.
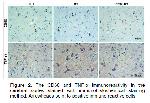
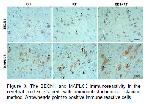
The formation of autophagosomes is the fundamental step of autophagy 19,41,42. A few autophagy-related genes and proteins are involved in this process and function in autophagosome formation 20. In the present study, the expression level of BECN1 and MAPLC3 proteins, which are autophagy-related proteins, were evaluated. BECN1, a key regulator of autophagosome formation, has an important role in autophagosome initiation and autolysosome maturation 43. MAPLC3 is a structural component of the autophagosomal membrane 44. In the present study, cell density showing BECN1 immunoreactivity was found to be significantly higher in the RT group compared to the control group. Also, in the RT group, it was determined that the cell density showing MPLC3 immunoreactivity increased, but this increase was not statistically significant compared to the control group. Besides the maintenance of cellular homeostasis, autophagy also functions as a response to cellular stress caused by exposure to a variety of chemical, and physical factors. It was documented that RT treatment also is one of the stress factors that induce autophagy in both cancer and normal cells 45. Similarly, in the current study, the increase in BECN1 and MAPLC3 expression due to RT administration indicates that RT induces autophagy. A previous study also reported that RT also induces autophagy and increases the expression of autophagy-associated proteins BECN1 and MAPLC3 46.
Increasing evidence has revealed that RES is the potential of promoting autophagy 16. It has been reported that RES, which is suggested to modulate various cellular processes, can stimulate autophagy through different signaling pathways. RES can directly activate autophagy by regulating mTOR signaling pathways, as well as can stimulate autophagy by deacetylating histone acetylases via AMPK/SIRT1 signaling and thus exert a neuroprotective effect 47. In the present study, it was found that RES administration significantly stimulated autophagy. BECN1 and MAPLC3 positive cell density were found to be significantly higher in the RES+RT group than in both the control and RT groups. A previous study has reported that the neuroprotective effect on microglia-mediated inflammation of RES is associated with promoting autophagy 48. As mentioned above, RES administration has been found to significantly reduce the RT-induced increased CD68 and TNFα positive cell density, as well as the degenerated neuron density.
Consequently, this study has indicated RES has neuroprotective effects against RT-induced neuronal tissue damage via its anti-inflammatory activities. Evaluating together all the data, these effects of RES were related to the inhibition of microglial activation by promoting autophagy, and subsequently the reduction of pro-inflammatory factors release
Acknowledgments: This research is a branch of study which has been supported by a research grant from the Scientific and Technological Research Council of Turkey (Grant number: 1919B012102187) and contains early results of this project.
Declaration of Competing Interest: The authors report no declarations of interest.