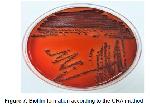
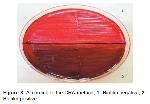
This study showed that the highest rate of biofilm formation with A. baumannii and P. aeruginosa isolates were detected by MP (Safranine) method.
NFGN bacteria are among the leading agents of community-based infections, particularly hospital infections 11. Nosocomial infections, especially in patients hospitalized in intensive care units, are difficult to treat and have high mortality. In cases with inappropriate antibacterial therapy, mortality rates of serious infections such as ventilator-associated pneumonia (VIP) and bacteremia reach 60% 12. The most important virulence agents of NFGN bacterial species are biofilm formation 13. Biofilm is an important structure for bacterial adherence, antibiotic resistance, and phagosytosis. The fact that bacteria come together to form a community and adapt to the environment through gene modulation (Quorum Sensing) has further increased the importance of biofilm structure 14.
There are different methods used to detect the biofilm formation. In our study comparing biofilm formation in NFGN bacteria with different methods (MP, ST, CRA) and investigating antibiotic resistance developing in the biofilm media, 150 NFGN bacterial strains isolated from various clinical samples were evaluated. The biofilm formation of 17 A. baumannii strains isolated from different hospitals in Turkey by Can F. et al. was examined by quantitative method and biofilm formation was observed in 9 of the isolates 15. In a study conducted by Wang et al., biofilm formation was investigated in 273 A. baumannii isolates using the MP method and 71 (26%) isolates were observed to be biofilm positive 16. Yassein et al. detected the presence of biofilms in 20 of the 50 P.aeruginosa strains isolated from clinical samples 17. Rodríguez-Baño et al. investigated the biofilm formation in 92 A. baumannii isolates by the microtitration plate method and found that 56 (63%) insolates formed biofilms 18. Delissalde et al. investigated the biofilm presence in P. aeruginosa isolates, isolated from 162 clinical samples using the MP method, and found 18% biofilm presence at the end of 24 hours of incubation 19. Abdi-Ali et al. detected biofilm formation at the rate of 80% with the ST method and 75% with the MP method in 75 A. baumannii isolates 20. Considering the distribution of biofilm positivity of clinical samples included in our study according to different methods, MP (Safranine) was determined to be 83 (55.33%), MP (Crystal violet) 61(40.6%) 6), CRA 71 (47.33%), ST (Safranine) 65 (43.33%), ST (Crystal violet) 57 (38%). Our study results are in line with other studies on the subject, and it is considered that the differing results are due to the fact that the biofilm formation process is a quite dynamic process, besides various environmental factors such as heat, temperature, pH, and nutrient concentration in the environment, biofilm formation is basically a similar process in general terms. However, it is considered that this may be due to the fact that it contains significant differences between the species and strains it contains in terms of formation steps.
In a study, the biofilm-forming properties of 60 A. baumannii isolates isolated from sputum, wound, and catheter samples were evaluated by the MP (crystal violet) method and it was determined that 10 (16.6%) of the 60 isolates had weak biofilms, 19 (31.6%) had moderately strong biofilm and 31 (51.6%) of them formed strong biofilm 21. In the study conducted by Babapour et al., 10.26% of 156 A. baumanii isolates were detected as biofilm positive by the CRA method, while the percentage of bacteria forming biofilm positive with ST, MP, and modified MP methods was determined as 48.72%, 66.66% and 73.72%, respectively 22. In another study, biofilm formation was qualitatively observed in 34 of 55 A. baumannii isolates, with the ST method, and strong biofilm formation was detected in 34 isolates and weak biofilm formation in 14 isolates by the MP method quantitatively 23. Our study with the MP method and ST method and two different dyestuffs used in these two methods is in parallel with other similar studies. In our study, biofilm formation was also evaluated by the CRA method, and 71 (47.33%) of the isolates were found to be biofilm positive and 79 (52.67%) biofilm negative. The majority of clinical samples in our study consisted of wound samples from the plastic surgery clinic (54%). NFGN bacteria are considered to have a higher risk of transmission from wounds than the other ways of transmission. Biofilm formation was determined in 47 (58%) of 81 wound samples by the CRA method and 54 (66.67%) by the ST (Safranine) method, and no biofilm formation was observed in the sterile body fluid samples. In our study, the highest biofilm positivity in A. baumannii and P. aeruginosa strains was determined by MP (Safranine) method, being 54 and 28, respectively. In addition, strong positive (+++) biofilm-forming strains were obtained with the MP (Safranine) method at the highest rate. Biofilm formation was detected in 44 (42.3%) of A. baumannii strains and 25 (59.5%) of P. aeruginosa strains by the CRA method. In the study conducted by Harika et al., the highest biofilm positivity rate in P. aeruginosa strains was obtained with the MP method, and either weak positivity or negative results were obtained with the ST and CRA methods 24. There are studies in which ST 25 and CRA 26 methods are not recommended to identify biofilm-producing isolates.
According to some studies conducted in our country, resistance to carbapenems, which are usually used as the first choice in treatment, has been detected at levels of 90-94% in Acinetobacter and 25-33% in Pseudomonas 27,28. In the study of Rao et al., biofilm positive A. baumannii isolates showed 100% resistance to IPM, 89% to cephotaxime, 80% to AK, and 73% to CIP 23. In our study, the imipenem resistance rates of biofilm-positive strains were 75% in P. aeruginosa and 100% in B. cepacia, while this rate was 85.19% in A. baumannii. According to these results, it is understood that carbapenem-resistant strains are more likely to produce biofilms compared to carbapenem-sensitive strains. While the imipenem resistance in our study was consistent with other studies for A. baumannii, it was concluded that the percentage of P. aeruginosa was higher than the ones in other studies. Resistance levels vary by geographic region. For instance, between 1997 and 2000, gentamycin resistance in P. aeruginosa was 15.8% in North America, compared to 28.3% in Europe and 38.2% in Latin America 29. These differences between the data obtained suggest that the studies were conducted at different times and in different locations, the clinics where the samples were isolated are different, and the bacteria are becoming more resistant by the day. Since resistance rates may vary depending on the location and usage times, it is a natural result that the resistance rate increases day by day. In our study, biofilm-positive A. baumannii strains were found to have high levels of resistance to all antibiotics, especially AMC (88.89%). In contrast, A. baumannii strains were shown to be sensitive to CT and SCF with a maximum of 35.18%. The study, conducted by Schaber et al., showed that biofilm-positive P. aeruginosa strains became approximately 10 times more resistant to imipenem, betalactam, gentamicin, and piperacillin-tazobactam antibiotics than the planktonic form 30. In our study, it was concluded that there is a positive correlation between biofilm formation and multidrug resistance in NFGN microorganisms. As colistin resistance increases in A. baumannii strains, an increase in biofilm production capacity is observed 31. In this study, colistin resistance was determined as 43.37% according to the antimicrobial susceptibility results determined by the disc diffusion method of biofilm-positive strains. This study indicated that biofilm-positive Pseudomonas strains showed the most sensitivity to CT (57.14%) and SCF (53.57%), while they showed the most resistance to AMC (92.86%). Dizbay et al. reported resistance rates as 49% for meropenem, 62% for ceftazidime, and 56% for SXT for B. cepacia strains, which they identified as nosocomial infection agents in their five-year study 32. In our study, it was determined that B. cepacia strains showed 100% resistance to all antibiotics except SCF (25%).
In conclusions today, the intensive or unnecessary use of antibiotics cause the formation of resistant bacteria. One of the leading causes of hospital infections, the non-fermentative gram-negative bacteria are protected from both host defense and antimicrobial agents by forming a biofilm, which may be considered as an important virulence factor. In this study, we have determined that the best method to show the formation of biofilm in NFGN bacteria is the MP (Safranine) method.
Conflict of Interest: The authors declare that there is no conflict of interest regarding this article.