Forty-four female adult Wistar Albino rats (200-250 g) were randomly assigned to four groups as follows: G1- Control (n=10), G2- Sham-operated (n=10), G3- Normal saline (n=12), G4- EGb-761(n=12).
All surgical procedures and sampling were performed under general anesthesia. Anesthesia was induced by intramuscular injection of ketamin HCL 12.5mg/100gr (Ketalar flk, Eczacibasi, Turkey) and Xylazine HCl 1mg/100gr (Rompon flk, Bayer). Laparatomy was performed with an average of 2 cm middle line incision under aseptic condition. In control group were applied no surgical procedure. In sham-operated group, only the common bile duct (CBD) was identified and mobilized but not ligated. In other treatment groups, common bile duct was dissected and ligated (CBDL) with 4/0 silk for twice and excised between the two knots in order to avoid recanalisation. Abdominal incision were closed with 3/0 silk sutures.
The rats in the control and sham groups received no medical treatment. Group 3 received 1 ml normal saline, Group 4 were given 50 mg/kg dose EGb-761 (Tebokan drops 50 ml Abdi Ibrahim, Turkey) via orogastric intubation twice daily for 2 days before and after the operation. Following this treatment, 1 ml of E.coli suspension containing 1 x 1010 bacteria in phosphate buffer saline was administered to each group through orogastric intubation on the postoperative day 2. Twelve hours after the administration of E.coli suspension, samples were obtained and the rats were sacrificed.
Following the laparatomy, blood samples were taken by cardiac puncture which were preserved at 20 ºC until assayed. Distal terminal ileum and the caudad (omental) lobes were excised and soaked in 10% formaldehyde for histopathological examination.
For PCR analysis, DNA was isolated from the blood samples of the rats using a commercial DNA extraction kit (Wizard Genomic DNA Purification System, Promega Corp., Madison, WI). The PCR protocols were used as described previously (17). Ten microliters of the amplification products were run on a 2% agarose gel and the product was visualized by ethidium bromide staining (Figure- 1).
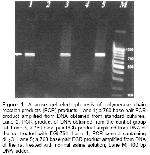
Click Here to Zoom |
Figure 1: Agarose gel electrophoresis of polymerase chain reaction products (PCR) products. Lane 1; a 760 base pair PCR product amplified from DNA obtained from standard cultures, Lane 2; PCR product of DNA obtained from the control group rat, Lane 3; a 760 base pair PCR product amplified from DNA of the rat treated with EGb761, Lane 4; PCR control containing dH2O. Lane 5; a 760 base pair PCR product amplified from DNA of the rat treated with normal saline solution, Lane M; 100 bp DNA ladder. |
During analyses, previously confirmed colonies obtained from standard cultures were used as positive control for E. coli. The sensitivity of PCR was determined with 10-fold dilution of the DNA extracted from the isolated colonies E. coli. In order to overcome both false positivity and negativity, we included negative and positive controls in each experiment.
Liver and terminal ileum tissue samples were fixed in formaldehyde solution and divided into 0.5 cm sections. After the standard laboratory proceedings, 5 µm slices were prepared for subsequent histopathological examination, by fixing the sections in paraffin and staining them with hemotoxiline-eosin. Liver tissue samples were examined by light microscopy and the results were scored in aspects hepatocyte bloating, multinucleation, degeneration, cell necrosis, Kupffer cell proliferation, cholestasis, portal edema, portal region extension, portal PNL infiltration, bile duct proliferation, bile duct dilatation and portal fibrosis: 0 (n/a), 1 (mild), 2 (moderate), 3 (intensive) and 4 (severe).
Terminal ileum samples were examined by light microscopy and the results were scored in aspects of mucosal damage, PNL infiltration in lamina propria, lenfoid follicular hyperplasia, goblet cell reduction, increase in the number of veins and capillary expansion: 0 (n/a), 1 (mild), 2 (moderate), 3 (intensive) and 4 (severe).
Serum urea, creatinin, total bilirubin, gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP) and alanin aminotransferase (ALT) were analyzed outomatically using a kit Olympus Diagnostica GmbH'dan (Wendenstrabe, Hamburg-Deutschland).
The data were given as avarage value ± standart error. For the statistical comparison of biochemical and histopathological results of trial groups, “Bonferroni adjusted Mann Whitney U test” was used and P<0.025 was considered to be significant. “Pronounced chi-square test” was used for the comparison of microbiological results and P<0.05 was considered to be significant.






