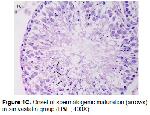
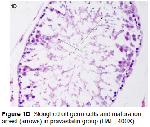
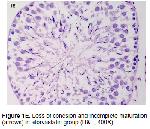
Testicular IR involves multiple pathophysiologic
mechanisms, such as reactive oxygen species
production
17,18, production-release of inflammatory
mediators
15,19 and neutrophil recruitment
20.
Oxidative stress plays an important role in testicular IR
injury
3,4 and antioxidant agents such as melatonin
14,21, allopurinol
22, lycopene
23, could limit
testicular IR injury. It has been reported that statins have
additional antioxidant, anti-inflammatory,
immunomodulatory, antithrombotic, vascular protective
and neuroprotective pleiotropic effects beyond their lipid
lowering effects lowering effects
24-27. In various experimental models of IR injury, it has been notified that
the protective effects of statins were shown after
prolonged pretreatment lasting a few days up to four
weeks
7-10.Recently, it has also been reported that
acute pretreatment with one or two doses administrations
of statins may be protective to IR-induced organ
damages, such as heart
28, kidney
8, brain
29 and
testis
11,12. The researchers have suggested that
these protective effects of statins are related to
upregulation of endothelial nitric oxide synthase (eNOS),
increasing the production/release of NO, reducing
inducible NOS (iNOS), and inhibition of NADPH oxidasedependent
superoxide anion production
28,30,31
In our study, we histologically evaluated the testicular
injury by the Johnsen’s score, and simvastatin (5 mg/kg),
atorvastatin (10 mg/kg) and pravastatin (10 mg/kg) were
administrated by single dose. Among these drugs, only
simvastatin provided significant improvement in IR
testes. Similar to our study, Yang et al.12 reported that
simvastatin protected testes from torsion-detorsion injury
in a dose dependent manner and histological findings
revealed severe injury in testes of the torsion-detorsion
and torsion-detorsion-simvastatin (1mg/kg) groups while
testes in the torsion-detorsion-simvastatin (5mg/kg)
group showed moderate injury. They suggested that
mechanisms of protective effect of simvastatin may
involve attenuating nuclear factor-kappaB activation and
decreasing oxidative stress induced by torsion-detorsion.
Karakaya et al.11 measured the blood flow of the testis
with laser doppler flowmeter in the experimental
testicular torsion model and observed that rosuvastatin
could protect the tissue perfusion.
Statins display remarkable chemical and
pharmacokinetic differences that are crucial for their
potential protective effects on IR-induced tissue
damages. Lovastatin, simvastatin and pravastatin are
fungal derived inhibitors of HMG-CoA reductase, while
atorvastatin, pravastatin, cerivastatin, fluvastatin,
pitavastatin and rosuvastatin are fully synthetic
compounds32. When the lipophilicity of statins that are
used in our study are compared; pravastatin is more
hydrophilic as a result of a polar hydroxyl group; and
although simvastatin and atorvastatin have similar
lipophilic properties, simvastatin is more lipophilic than
atorvastatin33,34. Lipophilic statins, such as
simvastatin and lovastatin, easily cross blood-brain
barrier by simple diffusion, whereas hydrophilic statins,
such as pravastatin and rosuvastatin, do not35. In our study, the protective effect which is presented by only
simvastatin may be related to its more lipophilic property
than pravastatin and atorvastatin, thus simvastatin may
easily across the blood-testis barrier, be compared to
others. Simvastatin is a prodrug and converts to its active
metabolites by phase I metabolism. Pravastatin and
atorvastatin are not prodrugs. Pravastatin is metabolized
to inactive metabolites while atorvastatin has active
metabolites36. In the present study, while atorvastatin
and pravastatin were administered 10 min before the
reperfusion, simvastatin, as a prodrug, was administered
30 min before. While simvastatin and pravastatin has a
short peripheral plasma elimination half-life (2-3 h and
1.3–2.8 h, respectively)37, atorvastatin has a longer
peripheral plasma elimination half-life (7 h), with a
prolonged inhibitory effect (20–30 h) resulting from the
contribution of its active metabolites38. In our study,
simvastatin presented protective effect, but it did not
reverse the scores back to normal levels. This condition
is related to its short elimination half-life. If it had been
administered twice instead of single dose, it would have
presented a more protective effect. Although atorvastatin
has similar lipophilicity with simvastatin, it did not present
the protective effect like simvastatin. In our study, since
atorvastatin was administrated 10 min before the
reperfusion, we thougt that atorvastatin might not be
sufficiently metabolized to its active metabolites which
have been responsible for most of its main effect.
In conclusion, in this study, while single dose
administration of atorvastatin and pravastatin did not
present the protective effect, simvastatin caused a
reduction of testicular damage during IR injury via
mechanisms independent of lipid lowering activity. These
results show that different statins present different effects
in testicular IR model, and this difference may be related
to remarkable chemical structures and pharmacokinetic
properties of these drugs.
Acknowledgments
This work was supported by a research fund from the
Zonguldak Karaelmas University (2008-01-01). The
authors thankfully acknowledge to Hasan Tahsin Yilmaz
and Bayram Çakan for caring of animals.
Conflict of Interest
No conflict of interest was declared by the authors.