RT has been the mainstay of treatment for NPC for more than three decades. Until early 1990s, the use of 2DRT to deliver a “tumoricidal” dose (66–70 Gy, 2 Gy per fraction for 6.6–7 weeks) to the target via laterally opposed fields had been the standard treatment modality. This technique involved the manual projection of tumor volume and organs at risk (OARs) onto the orthogonal simulation films based on bony anatomy and the employment of nonconformal shielding blocks to protect the critical structures. The obvious drawbacks include normal tissues that lead to additional treatment complications and compromise of target coverage thus leading to local failures.
7,8. LC rates of 71–93% for stage T1–2 and 40–68% for stage T3–4 disease have been reported for conventional RT techniques with or without concurrent chemotherapy
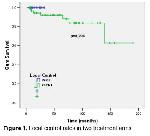
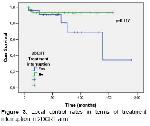
9-11 . In the current study, 2-, 3-, and 5-year LC rates in the 2DCRT patients were 94.3%, 92%, and 92%, respectively. LC may be improved with an increase in the radiation dose or with concurrent CTX
12,13. However, the dose to the primary tumor is limited by the tolerance of the adjacent normal structures, especially when involvement of the base of the skull or intracranial spread is present. The incidence of severe and life-threatening toxicity of combined conventional RT and CTX was 55% for grade 3 and 21% for grade 4 in an intergroup trial for NPC.
13 . Morretto et al.
14 demonstrated that rates of 8% for acute skin toxicity ≥G3, 23% for mucositis ≥G3, and 19% for dysphagia ≥G3 in the 2DCRT and 3DCRT arms. In our study, grade 4 toxicities were noted in patients treated with 2DCRT. The rate of acute xerostomia ≥G3 was 74.6%, and the rate of mucositis ≥G3 was 7.3% in the 2DCRT arm.
The transition from 2D-RT to 3DRT, in particular IMRT, represents a major step forward in the treatment of NPC. Unlike 2DCRT, IMRT CT planning exploits the spatial relationship between targets and OARs and allows for more comprehensive irradiation of the tumor and greater protection of critical structures. Although a number of dosimetric studies demonstrate an advantage of IMRT over 2DCRT in the treatment of NPC15-189, further clinical data are still needed. Sultanem et al.19 has achieved excellent LC with IMRT use in treatment of NPC in a study of 35 patients. IMRT delivers high doses to the tumor while protecting critical organs, such as salivary glands. Furthermore, previous studies demonstrated that IMRT leads to increased tumor doses and increased normal tissue protection compared to 3-D conformal planning20,21. A study of 86 patients (26% treated with IMRT) revealed a LC, and locoregional control LRC, and an OS or 96%, 93%, and 90%, respectively22. Patients were stage III–IV in 75% of these cases, and 70% of patients were treated with induction plus concurrent CTX, while 20% were treated with concurrent CTX only. In the current study, the 2-, 3-, and 5-year LC rates for the patients treated with 2DCRT were 100%, 100%, and 100%, respectively.,
In a study by Morretto et al.14, acute skin toxicity ≥G3 occurred in 15%, mucositis ≥G3 occurred in 31%, and dysphagia ≥G3 occurred in 31% of the patients treated with IMRT. In the study, life-threatening grade 4 toxicities were not observed in the patients treated with IMRT. In our study, rates of acute xerostomia ≥G3 were 78.3%, while mucositis ≥G3 occurred in 21.7% of patients in the IMRT arm.
The total radiation dose, as well as the fractionation and overall treatment time OTT, are decisive for local and regional tumor control for non-NPC head and neck patients23,24. With conventional fractionation, a break of about 1 week is associated with an absolute reduction of 10–12% in LC rates. A break of even 1 day could reduce the LC rate by about 1.4% regardless of the fractionation schedule or primary tumor site; however, this type of information is limited in NPC patients. Researchers from Hong Kong Queen Mary Hospital first reported an adverse effect of a treatment break for NPC on locoregional control and disease-free survival. Patients with prolonged OTT fared worse in terms of locoregional control, distant metastases-free survival, and disease-free survival. The negative effect of a treatment break was not offset by the use of an additional boost.25 Xu et al.26 suggested that treatment break is an independent prognostic factor associated with long-term survival in patients with NPC. In this study, 177 of 1706 cases (10.4%) had a treatment interruption of more than oneweek. Interruption of RT for more than 7 days is associated with an 18% reduction in 5-year survival rates16.
In our study, a conventional fractionation schedule was used for almost all patients treated with 2DCRT, resulting in a median OTT of 58 days. On the other hand, a slightly hypofractionated accelerated schedule was chosen in most IMRT cases (median OTT 48 days). Treatment times in both groups were statistically significant. However, In our study, there was no statistically significant difference between 2DCRT and IMRT regimen, in terms of LC, acute and late toxicity. In our study, 27 patients (34.6%) interrupted their RT treatments due to acute toxicities, and median treatment time was 3 days (range, 2–14). In the 2D-RT arm specifically, the median treatment interruption time was 3 days (range, 2–14), while in the IMRT arm, the median treatment interruption time was 6.5 days (range, 3–10). When interruptions were evaluated in terms of treatment time in the 2DCRT arm, 23/55 patients (41.8%) interrupted their treatment, and in the IMRT arm, 4/23 patients (17.4%) interrupted their treatments. Although the patients in the IMRT arm appeared to be more compatible with the treatment than those in the 2DCRT arm, this difference was not statistically significant.
Xerostomia is the most common late-stage side effect of RT for head and neck cancer27 and is the most common problem following RT for NPC. In astudy of 934 NPC patients treated with 2D RT alone, Chen et al28 reported that the 5-year incidence rates for radiation- induced brain injuries, trismus, hearing loss, and xerostomia were 1.5%, 13.6%, 31.1%, and 38.7%, respectively. In contrast Wang et al.29 reported that incidence rates for radiation- induced brain injuries and trismus were only 0.8% and 1.1%, respectively.
In this study, the incidence rate of late xerostomia was 50.9% and was 2.7% for grade 3 late xerostomia in the 2DCRT arm. The rate of soft tissue fibrosis was 36.4% in 2DCRT arm. The use of IMRT has enabled sparing of the parotid glands, resulting in significant reduction of the incidence of xerostomia. Nancy et al.30 revealed that the rates of G1 and G2 xerostomia in NPC patients 3 months after IMRT treatment were 35% and 65%, respectively, and at 12 months after the treatment, these rates changed to 50% and 0%, respectively. In our study, the rate of late xerostomia was 43.5% in the IMRT arm. Tissue fibrosis occurs in the neck region due to high doses of radiation. Sham and Chow31 declared that tissue fibrosis occurred in 9% of the patients in their series. In our study, fibrosis occurred in 36.4% of the patients in the 2DCRT arm and in 56.5% of the patients in the IMRT arm. Overall, no statistically significant differences were detected between the 2DCRT and IMRT treatment arms in terms of chronic toxicities.
Many factors are associated with the results of NPC treatment. The following factors are generally thought to affect the results of RT treatment of NPC: gender, age, anemia, T stage, N stage, M stage, histopathology, RT dose, RT field, and inclusion of CTX32-34. Regarding chemoradiation, optimal doses and sequencing (i.e., neoadjuvant, concurrent, and adjuvant approaches) remain controversial. In our study, the median age (46 years) and the male/female ratio (2.7/1) were the same as in most clinical trials5. The proportion between early and locally advanced disease (78% of stage III–IVB) is similar to other previously published studies22. In our study, no significant prognostic factors that have an effect on LC were identified.