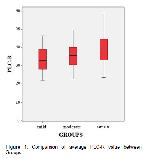
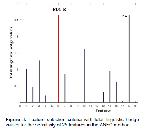
The critical result of our study is that PLC-R was remarkably higher in the severe COVID-19 patients. According to different types of correlation analysis results, PLC-R levels were positively correlated with COVID-19 severity.
Inflammation caused by viral infections can cause dyslipidemia in patients. A previous study reported that the LDL-c levels of COVID-19 patients decreased 14. SARS-CoV-2 can damage, liver function and reduce LDL-c biosynthesis. SARS-CoV-2 causes acute inflammation by causing an increase in proinflammatory cytokine levels. It can disrupt the efflux and transport of lipids by changing liver functions in cytokines. Free radicals occurring in the host cell in viral infection can also disrupt lipid metabolism 15. SARS-CoV-2 infection can cause cholesterol molecules to leak into tissues such as alveolar spaces to form exudate by increasing vascular permeability. This causes the lipid level in the plasma to decrease 16. This study observed that LDL-c levels decreased in the severe Group of COVID-19 patients.
The literature studies found severe impairment occurred in kidney function tests and blood electrolyte levels in severe COVID-19 patients. Acute kidney damage due to cell and tissue damage from SARS-CoV-2 and lack of oral intake of patients reveasl these pathological findings 17. According to our data, as the severity of COVID-19 infection increased, urea, Na, Ca levels increased meaningfully.
An increase in non-specific inflammatory biomarkers such as CRP, D-dimer, and ferritin is observed in COVID-19 patients. A study of 653 COVID-19 patients reported that serum ferritin values in patients with severe COVID-19 infection were higher than patients with mild COVID-19 infection 18. Shah et al. reported no significant difference in serum ferritin levels and transferrin saturation between patients with non-severe and severe hypoxemia, but serum iron levels were significantly lower 19. In our study, in the Group with severe COVID-19 infection, it was observed that CRP and ferritin levels increased statistically, and iron levels were statistically decreased. There was no significant difference in transferrin saturation. High ferritin levels may indicate a robust inflammatory response, with SARS-CoV-2 entry into the human body and its effect on iron metabolism 20. Iron is an essential micronutrient for both humans and pathogens 21. During infection, humans' innate immune system response limits the amount of iron to deprive it of the pathogen, leading to anemia 22. Anemia reduces oxygen delivery to tissues and causes multi-organ failure. Studies show that Hb levels decrease in COVID-19 infection 23. We also obtained findings that support previous studies. Inflammatory changes caused by COVID-19 infection can affect erythropoiesis and cause a decrease in hemoglobin. SARS-CoV-2 binds to the beta chains of hemoglobin via surface glycoproteins, inhibiting both metabolism and hemoglobin denaturation. Hemoglobinopathy and iron dysmetabolism together with hypoxia can seriously compromise the O2 carrying capacity of erythrocytes and induce tissue changes due to hyperferritinemia 24. For these reasons, decreased hemoglobin levels for COVID-19 may be an indicator of disease progression.
Viral and bacterial infections are generally associated with thrombocytopenia and changes in platelet morphology. Platelets are involved in hemostasis, coagulation, immune, and inflammatory responses 25. SARS-CoV-2 uses the spike protein to enter host cells by binding to angiotensin-converting enzyme 2 in the host cell membrane. Spike protein enhances thrombus formation. Coagulation factors are released, inflammatory cytokines are secreted, and leukocyte platelet clusters are formed 26. Fibrin deposition with coagulation activation and subsequent fibrinolysis increase D-dimer level, an essential intravascular coagulation indicator. Studies have proven that high D-dimer values at admission to the hospital are associated with poor prognosis 27. According to the results of our research, it was observed that D-dimer values increased statistically significantly in severe COVID-19 infection. Continued inflammation can rapidly lead to the development of cytokine storm and macrophage activation syndrome and hyperinflammatory response. Cytokine storm is a syndrome that releases proinflammatory cytokines that cause acute respiratory distress syndrome and multiple organ dysfunction syndromes in case of infection 28. Macrophage activation syndrome is a proinflammatory cascade associated with a high rate of thrombosis and death in sepsis 29. In COVID-19 disease, increasing cytokine levels and inflammation initiate platelet destruction by causing bone marrow involvement. Accumulation in the lungs causes more platelet consumption. The decrease in the platelet count leads to an increase in the production of young and immature platelets that are functionally more active and larger sized 30. Larger platelets have more granules and receptors, and they are metabolically and enzymatically more active than small platelets, produce more thromboxane A2, and tend to clot more quickly 31. Since the destruction in the bone marrow will be more significant in severe COVID-19 infection, the number of large platelets released into the circulation will increase with the release of more megakaryocytes 32. Mitophagy impairment occurs in patients with COVID-19 due to oxidative stress and hyperinflammation. Mitophagy protects platelets from oxidative stress and mitochondrial destruction by removing damaged mitochondria to prevent platelet apoptosis in healthy individuals. However, mitochondrial dysfunction in platelets in COVID-19 infection may affect platelet survival and apoptosis, potentially increasing the risk of thrombus formation. Studies have shown that apoptotic platelets can induce clotting ≥50 times faster than normal platelets 33. In the studies, patients with myocardial infection have reported that larger platelets contribute to the prothrombotic state 34. Microvascular and macrovascular thromboembolic complications in the lung, spleen, brain, intestine and peripheral vessels have been reported in COVID-19 35. It has been reported that stroke has been detected in young patients with covid-19, even without underlying coagulopathy before the infection 36. Large platelets may be one of the causes of these thrombotic events seen in COVID-19 infection.
Platelet activity should be evaluated with PVI parameter values, not platelet counts. PVI parameters are measurements calculated by devices and can be easily detected in blood tests, typically as part of the CBC, not requiring advanced or expensive technology 11.
The MPV and PDW parameters are widely and routinely used in clinics around the world. When platelet production decreases, young platelets grow, their volume increases, and they become more active. As a result, MPV and PDW levels increase 37. It has been reported that MPV and PDW values increase to higher levels in patients with sepsis, and PDW is a poor prognostic factor in severe sepsis 38. In a study by Güçlü et al., it was reported that mortality increased in parallel with the increase in MPV value in COVID-19 infection 39. However, we found that PLC-R was significantly higher in severe COVID-19 disease, although we did not see an increase in these values. In the meantime, a comprehensive calculation was made with the ANFC method, while PLC-R findings were found as a constant factor parameter, and it was brought to the literature.
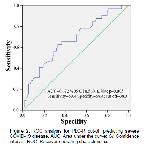
PVI in patients with COVID-19 infection is a simple, effortless, and economical test that should be used to predict the probability of acute thrombotic events. Larger platelets can easily be identified during routine complete blood count analysis, and such patients can benefit from preventive treatment. According to our data, it has been proven by many analysis methods that the PLC-R value can be used as a suitable biomarker that can describe the severity of COVID-19 infection.