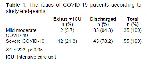Our study results showed that UII levels were significantly higher in both mild/moderate COVID-19 and severe COVID-19 patients compared to the control subjects. Moreover, there were more prominent increases in the severe COVID-19 patients and in patients who died or were transferred to ICU. In the correlation analysis, it was determined that the UII levels only correlated with the CRP levels. Additionally, it was determined that UII had a sensitivity of 80% and specificity of 59% in the differentiation of severe COVID-19 patients from mild/moderate COVID-19 patients.
UII is known to be the most potent vasoconstrictor, and it has many physiological and pathophysiological activities, such as vasoconstrictor and vasodilator actions, cell proliferation, pro-fibrosis, and inflammatory effects. UII is involved in the process of inflammatory injury and plays a prominent role in the onset and development of inflammatory diseases. Although urotensin and its receptor are much more abundant in the central nervous system, it is expressed in the various tissues, such as the lung, kidney, spleen, and small intestine in addition to circulating in human plasma 6,14. Particularly, the highest expression of urotensin mRNA was observed in the mice lung 15, which suggested a role of UII/UII-related peptide in respiratory function. In another study, consistent with this hypothesis, it was demonstrated that UII in vitro induced a concentration-dependent contraction of airways and pulmonary blood vessels in monkeys 16. Furthermore, Kristof et al. described the cellular distribution of the urotensin II system in the normal lung 17. Accordingly, it was demonstrated that U-II played active roles in several lung diseases, such as pulmonary hypertension, lung adenocarcinoma, and sepsis-induced lung injury. Previous studies also demonstrated that chronic hypoxia induction resulted in enhanced contractile activity in the airways and enhanced pulmonary responses to UII in rats 16,18. Moreover, increased UII secretion with hypoxia was demonstrated in both in vitro and in vivo analyses 19,20. Same as chronic hypoxia, acute hypoxia results in increased UII secretion 21. Contrary to this response in rats, UII did not exhibit this response in pulmonary arteries isolated from humans 18,22. In another study, the cellular localization of UII and URP in patients with lymphangioleiomyomatosis was defined 17. On the other hand, synthetic UII stimulated A549 cell proliferation and accelerated the growth of A549 tumor xenografts in mice 23. Additionally, it was demonstrated that UII mRNA and UII immunoreactivity were also present in human lung adenocarcinoma cell line A549.These results suggest that UII may contribute to the pathogenesis of lung adenocarcinoma. UII promotes the release of IL‐6, TNF-α, and MMP‐9 (speculated to be due to the increased activation of NF‐κB) in the tumor microenvironment, and this may be one of the most important mechanisms by which UII promotes lung adenocarcinoma growth 24. In another study, the administration of UII resulted in increased expression of TNF-α, IL1 β, and IL6 mRNA in human endothelial cells 25. Additionally, it was reported that UII and its receptor contributed to the sepsis-induced lung damage of chronically diabetic mice, and this damage was prevented by the urotensin-II receptor antagonist 12. Many previous studies reported that UII antagonists reduced the inflammatory cytokine response 26-28. Dose-dependent reduction in the mRNA expressions of TNF-α, IL1β, IL6, and NFκB with UII antagonist in mice lung tissue and reduced serum TNF-α level were also reported in another study 12. For these reasons, it can be considered that UII modulates the inflammatory response.
All the evidence collected until today indicates that increased inflammatory response plays an important role in COVID-19, and the severity of COVID-19 is increased by the inflammatory cytokine storm. Accordingly, cytokines and chemokines lead to the accumulation of immune cells and activate the immune responses 29. For these reasons, it is important to determine the markers to monitor the progression of the disease and ensure the early treatment of patients. An anti-inflammatory treatment approach may play an important role while controlling the inflammation in the progression of COVID-19 1. To date, several inflammatory factors, such as IL-6, TNF-α, CRP, and PCT, were determined as independent factors in the prediction of the severity of COVID-19 30-32. In this study, the results demonstrated increased levels of UII in COVID-19 patients, especially in severe cases, and it was observed that UII was correlated with CRP. To our knowledge, this is the first study that demonstrates the increased levels of UII in COVID-19 patients and the correlation between UII and CRP. CRP is a sensitive systemic marker, which can be used as an indicator of inflammation. This study is preliminary in terms of demonstrating that UII can be involved in the pathogenesis of COVID-19, and it may be a potential biomarker for monitoring the progression of the disease. However, the relationship between U-II and the inflammatory markers, such as IL-6 and TNF alpha, were not evaluated in this study.
This study has some limitations. Firstly, our sample size was relatively small, and the study was conducted in a single healthcare center. Secondly, the lack of follow-up and post-treatment data may play significant roles while determining the significance of UII in this disease.
In conclusion, increased levels of UII may be related to disease severity in COVID-19 patients. Initial evaluation of UII in these patients can help predict the adverse outcomes. In future studies, larger sample sizes and participation from several healthcare centers can determine the role of UII on COVID-19 pathogenesis and treatment approaches.
Acknowledgments: This work has not received any financial support.