Research and Publication Ethics: This research was carried out in Gaziantep University Experimental Animal Research Center (GAUNDAM) in accordance with the ethical rules after it was approved by the Gaziantep University local ethics committee with decision number 2021/59 and protocol number 229
Experimental Design: This research was carried out at the Gaziantep University Experimental Animal Research Center in accordance with the ethical rules. The Gaziantep University Experimental Animals Research Center provided Sprague Dawley male rats (810 weeks old, 200 ± 20 g weight, n = 35) for this experiment. We kept the animals in the animal room for a week to allow them to get used to their new surroundings. Throughout the experiment, the animals were given ad libitum access to pellet feed and drinking water. All animals were housed in cages with a 12-hour light/dark photoperiod in automated air-conditioned rooms with a constant temperature of 21±2°C.
The animals were grouped as follows:
Control group: The animals were not given any intervention for five weeks.
Sham group: For five weeks (weekly at around 10:00 a.m. on Mondays), a physiological saline solution was given intraperitoneally (i.p.). For five weeks, a diluted DMSO solution was given intragastrically (daily at around 5:00 p.m.).
CIS group: For five weeks (weekly at around 10:00 a.m. on Mondays), a single dose of cisplatin (3 mg/kg) was given i.p. 15.
CIS+LSA group: For five weeks (daily at around 5:00 p.m.), SA (20 mg/kg) was given intragastrically [16, 17]. For five weeks (weekly at around 10:00 a.m. on Mondays), a single dose of cisplatin (3 mg/kg) was given i.p. (15).
CIS+HSA group: For five weeks (daily at around 5:00 p.m.), SA (40 mg/kg) was given intragastrically 16,17. For five weeks (weekly at around 10:00 a.m. on Mondays), a single dose of cisplatin (3 mg/kg) was given i.p. 15.
Drugs and Chemicals: All chemicals were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). SA was dissolved in dimethyl sulfoxide (DMSO) 12, and the DMSO was then diluted with physiological saline. The final volume of DMSO administered to the animals did not exceed 10%, and the animals were given this volume for five weeks. Cisplatin was dissolved in physiological saline. All solutions were freshly prepared before the experiment.
In Vivo Experiments
The rotarod motor activity test: The rotarod motor activity test is a frequently used technique for assessing motor coordination (Ugo Basile, Comerio, Italy). After acclimating the animals to the device and environment, a ramp protocol was used, beginning at 5 rpm and gradually increasing to 40 rpm for 300 seconds 18,19. The animals ran on the rotarod equipment 3 times, with a 5-minute break between each run. Group averages were then computed from the time spent by the animals on the rod.
The Hot/Cold plate test: This test was used to evaluate thermal hypoalgesia or hyperalgesia. A plate encircled by plexiglass cylinders (Ugo Basile, Comerio, Italy, model 35100) was heated to 55°C. On the hot plate, the animal was left alone. The time interval between when the animal was put on the plate and when its feet were withdrawn was measured. Rats were not allowed to remain on the plate longer than 30 seconds (cut-off) to avoid tissue injury and/or excess suffering, despite the fact that their usual response time is between 5 and 20 seconds 20,21. The cold plate test was performed to determine the presence of cold allodynia and cold hyperalgesia (model 35100, Ugo Basile, Comerio, Italy). Cold allodynia was evaluated at a temperature of 10°C, whereas cold hyperalgesia was assessed at a temperature of 4°C 22,23. Rats were only left on the plate for 50 seconds to avoid tissue injury.
The Tail-Flick test: The tail-flick test was used to determine the central effects of the pain threshold. For the tail-flick test, a photosensor was positioned beneath the area where the tail is inserted. The rat pulled its tail when it felt pain due to the thermal light (235 mW/cm2 and 50°C temperature) generated by the photosensor area at a constant distance from the 34 cm of the animals tail end in a special chamber (Ugo Basile 37360 Comerio, Italy). The duration between when the thermal light is applied and when the tail is pulled was then noted. To ensure that the animal would not suffer excessively, the duration was limited to 10 seconds 20.
The von frey filament test: This technique is used to determine the mechanical pain threshold. The rats were contained in an apparatus coated in plexiglass and supported by a metal perforated wire base. To assess the withdrawal threshold, a mechanical stimulus (von Frey filaments [0.415 g, logarithmically incremental stiffness; Bioseb, Vitrolles, France]) was applied to the hind paw, mid-plantar surface of each rat. Three times in series, the animals hind paw withdrawal responses were measured, with a minimum of 10 seconds between each measurement. The mechanical pain threshold was determined as the average of these 3 measurements 24-26.
The adhesive removal test: The adhesive removal test was used to assess sensorimotor function. Animals were expected to detect and remove rectangular labels (Kinesio bands) (10x10 mm) placed on the plantar surfaces of both forepaws 27. For this purpose, after the adhesives adhered to the plantar surface of the forehand, the rats time to notice and remove these adhesives was measured.
Following each behavioral observation, the cylinder and plate surfaces were carefully cleaned with 70% ethyl alcohol to eliminate any scent clues left by the previous animal.
Electrophysiological Experiments: The animals were anesthetized (with a combination of 10 mg/kg xylazine and 80 mg/kg ketamine) after the behavioral experiments were completed 28. The body temperatures of the animals were monitored utilizing a rectal probe, and the animals body temperatures were maintained at 3637°C via the heating plate.
Measurement of Nerve Conduction Velocity: Each animals right sciatic nerve was stimulated non-invasively with an animal stimulation electrode (AD instrument, product code: MLA0320) from 2 points 10 millimeters apart (proximal and distal) (Powerlab 4/25 t-276 models). The frequency of the electrical impulse was adjusted to 0.5 Hz and the duration to 0.1 ms. The negative electrode was then inserted at the greatest diameter of the gastrocnemius muscle, the positive electrode was inserted at the hindfoot 2-3 interosseus muscle, and the reference electrode on the opposite extremities. To determine the conduction velocity of the motoric branch of the sciatic nerve, the difference between the latency time of the compound muscle action potential (CMAP) induced by stimulation given from the proximal point and the latency time of the compound muscle action potential after stimulation given from the distal point was divided by the distance between the 2 points. In addition, the amplitude of the CMAP was measured from peak to peak. The following variables are required to measure nerve conduction velocity; range: 20uV, low pass; 2 kHz, high pass; 0.3 Hz, anti-alias; off, rate; 40 k/s, Powerlab; 4/25 t-276 models, software; LabChart v8.1.16). The MNCV was reported in meters per second 29.
Biochemical Analyses: After the nerve conduction velocity studies were completed, all rats were anesthetized with a combination of ketamine hydrochloride (80 mg/kg, (Pfizer Ilac, Istanbul, Turkey) and xylazine hydrochloride (10 mg/kg, Rompun; Bayer Turk Ilac Ltd., Istanbul, Turkey). Then, blood was taken from the inferior vena cava with a 5 mL injector under anesthesia. Blood samples were maintained for around 20 minutes for coagulation at room temperature. The blood samples were centrifuged at 4000 rpm at +4°C for 10 minutes, and sera were separated. We separated serum samples into Eppendorf tubes, which were then kept at -80°C until analysis. And then rat's sciatic nerves harvested. After that, the tissues were treated with Phosphate Buffer Saline (PBS) (PH 7.4). The tissues were then weighed using a precision balance, and PBS was added 9 times their weight (fresh tissue (gr)/PBS (mL): 1/10). The tissues were then homogenized in PBS with a tissue homogenizer and centrifuged at 5000 RPM at +5 oC. The supernatant samples were collected and kept in labeled Eppendorf tubes at -80 °C until the study day.
Determination TAC, TOC and index of oxidative stress (OSI): TAC and TOS levels were determined using a colorimetric technique developed by Erel 30,31. The measurement was carried out using a Beckman Coulter AU480 autoanalyzer with a commercially available TOC kit (Rel Assay Diagnostics, Gaziantep, Turkey), and the findings were calculated as μmol H2O2 Eq/L. The ratio of TOC to TAC was accepted as the Oxidative Stress Index (OSI). Calculation of the OSI; It was calculated as an arbitrary unit using the formula TOC (μmol H2O2 Eq/L)/(TAC (mmol Trolox Eq/L) x 10) 32.
Determination of TNF-α, IL-6, IMA levels: The levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and ischemia-modified albumin (IMA) in the serum samples were determined with the enzyme-linked immunosorbent assay (ELISA) method using commercial kits (FineTest, Cat. Nos: ER1393 [TNF-α], ER0042 [IL-6], ER1108 [IMA], China). The color intensities were measured using an ELISA reader at 450 nm (Biotek ELx800, USA).
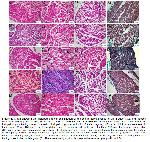
Histopathological Evaluation: The right sciatic nerve was isolated, and 1 cm of nerve tissue was fixed with formaldehyde solution (10%) for 24 hours and then made ready for blocking in the tissue tracking device. Then, 5μm thick sections were taken from the materials obtained from paraffin blocks in a microtome device, deparaffinized, and examined under a light microscope by staining with Hemotoxylin-Eosin (H-E) (200x and 400x). In addition, sections with a thickness of 5μm for histochemical staining will be deparaffinized, then stained with a Masson-Trichrome stain. A pathologist who was blinded to the study evaluated stained slides under a light microscope (Olympus BX51, Tokyo, Japan) (200x and 400x).
Influence of Sinapic Acid on the Cytotoxicity of Cisplatin In Tumor Cells
Adenocarcinomic human alveolar basal epithelial cells cancer cell line (A549) and a human non-tumorigenic lung epithelial cell line (BEAS-2B):
The effect of Sinapic acid on cisplatins antitumor activity was evaluated using an adenocarcinomic human alveolar basal epithelial cells cancer cell line (A549) and a human non-tumorigenic lung epithelial cell line (BEAS-2B). In our study, lung adenocarcinoma cell line A549 (American Type Culture Collection, LGC Standards GmbH, Wesel, Germany), pleura (BC-3), and normal bronchial epithelium (BEAS-2B) cell lines transformed with simianvirus40 (American Type Culture Collection, LGC Standards GmbH, Wesel, Germany) were used. A549 and BEAS-2B cell cultures were obtained as previously described in RPMI-1640 medium containing 10% FCS, 100 I.U/ml penicillin, and 100 μg/ml streptomycin. The cells utilized in the studies were cultured in a 75 cm2 culture vessel (Falcon; BD Biosciences, USA) equipped with an incubator set to 37°C, 5% CO2, and 95% humidity. 5 mL HBSS was used to wash the cells (Hank Solution Balanced Salt Solution).
Assay of cell viability: The viability of the cells was evaluated by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide) method. Briefly, the culture medium was replaced with serum free medium containing 1 mg/ml MTT (Sigma) and incubated at 37ºC for 30 min. Then the MTT solution was poured and DMSO (Sigma) was placed on the cells. The change in color was read at 550 nm with a colorometric reader (spectrophotometer) 33,34.
Cytotoxicity evaluation using MTT viability assay: Sinapic acid was dissolved in dimethylsulfoxide (DMSO, Sigma) and cisplatin was dissolved in physiological saline and prepared for cytotoxicity experiments at the concentrations determined in serum-free ('serum free, SF') medium. According to reference publications, 1, 3, 10, 30, 100 μM cisplatin doses will be used to determine the toxic dose of cisplatin 35. A549 and BEAS-2B cells were cultured in 96-well plates until they covered 70-80% of the surface, and then they were exposed to cisplatin at the determined doses. It was determined that cisplatin killed more than 50% of cells in post-exposure cancer cells (A549) but did not affect healthy cells (BEAS-2B). Similarly, the non-toxic dose of sinapic acid was determined by performing cell viability studies at different doses (1, 3, 10, 30, 100 μM) only on healthy cells (BEAS-2B). We investigated sinapic acid's effect on cisplatin's antitumoral activity to see whether it may be administered to prevent cisplatin-induced peripheral neuropathy. The determined cisplatin and sinapic acid doses were evaluated in cell lines (A549) and (BEAS-2B).
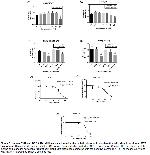
Statistical Analysis: The statistical Package for the Social Sciences (SPSS) Version 23.0 (SPSS Inc., Chicago, USA) package program was used to perform statistical analysis in this study. The Shapiro-Wilk test was performed to assess the normality of data distribution. For the normally distributed data, one-way variance analyses (ANOVA) were utilized. Following that, the Tukey posthoc test was used to establish which group the difference stemmed from. The descriptive statistics for numerical variables were expressed as group mean±standard deviation. The significance level was set at p<0.05. Finally, the graphics were drawn using OriginLab (OriginPro 2018 (64-bit; SR1 b9.5.1.195).